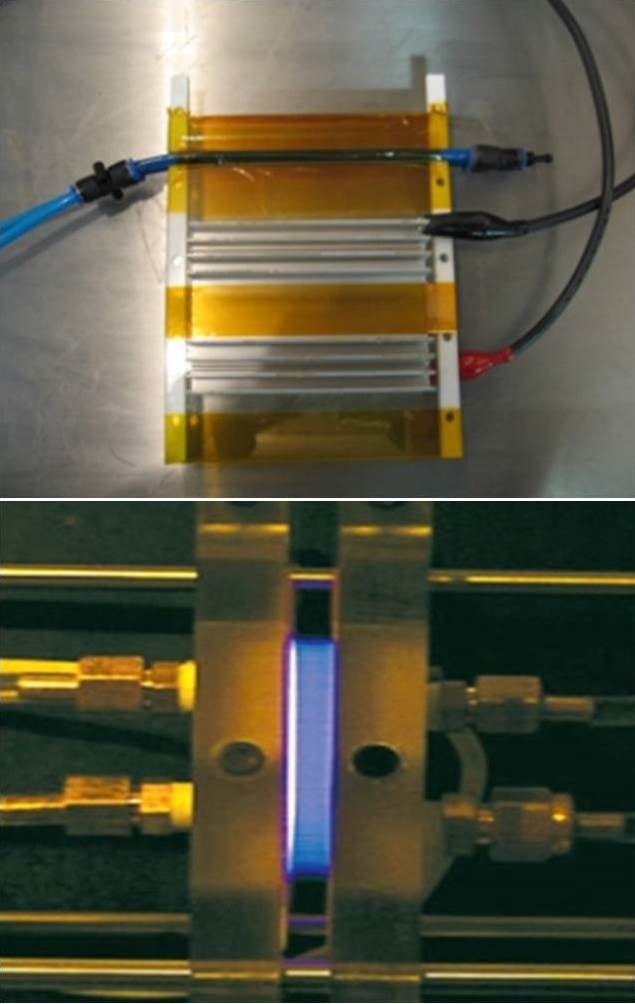
Cold plasma discharges have been proved effective against foodborne pathogens damaging cell membrane and wall and maybe used for decontaminating surfaces of food products.
Cold plasma treatment systems send an electrical power through a gas or gases, creating a plasma field and reactive molecular species.
Cold plasma systems typically have four components; Cold plasma discharge chamber, high voltage AC power supply, electrical parameter measurement and control devices, and
gas flow control devices.
Equipment varies with different electrodes, power supplies (e.g., frequencies), and applied pressures, and gas combinations needed for different applications and products.








Plant materials, including fruits and vegetables, provide a source of polysaccharides, proteins, and lipids. The use of plant-based material is favored to synthetic polymers due to its nature-friendly biodegradability. The increase in non-biodegradable waste material and its difficulty to be recycled have resulted in the development of new biodegradable materials suitable for packaging, including food packaging. Plant biopolymers are favored to animal-based proteins (e.g., collagen) used in edible films and coatings due to the consumers¡¯ interest in healthy food materials.
Heterogeneous biopolymers or biopolymer aggregates/particles in natural biomaterials not easily stabilized in water need to be broken down so that the natural biomaterials can form uniform matrices for packaging materials. Emerging technologies using high-pressure homogenization, ultrasound, irradiation, and enzymes could be used to prepare package-forming solutions with small enough biopolymer materials which allow forming packages with appropriate tensile, barrier, and optical properties.



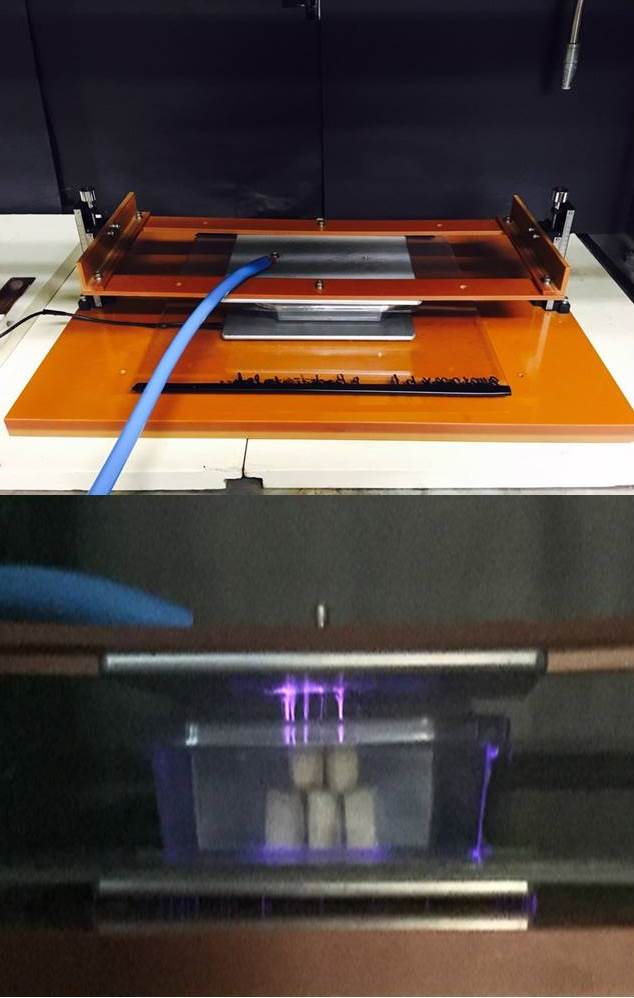
PEF treatments use high intensity electric field pulses generated between two electrodes.
The nonthermal treatment is attained by minimizing temperature increase in treatment zones using a very short pulse width of treatment time (i.e. microseconds).


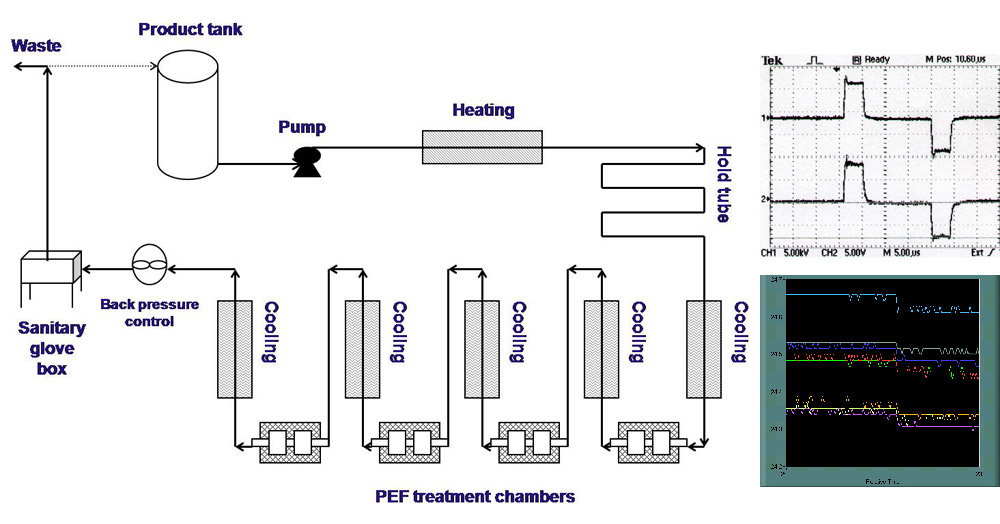
A PEF processing system consists of a high voltage pulse generator, a PEF treatment chamber, a data logging system, and a fluid handling system. The pulse generator, connected to the treatment chambers, generates electric pulses. PEF is delivered to foods inside the PEF treatment chamber.
PEF inhibits microorganisms by damaging microbial cell membranes. This damage leads to ion leakage, metabolite losses, protein releases, and increased uptakes of drugs, molecular probes, and DNA.
The factors determining the efficiency of the microbial inhibition by PEF can be classified with treatment parameters, product parameters, and microbial characteristics. The main treatment parameters that affect microbial inactivation by PEF are electric field strength, PEF treatment time, pulse width, pulse shape, and treatment temperature. The most critical product parameters include electric conductivity, density, viscosity, pH, and water activity. The type of microorganism, microbial cell size or shape, growth stage of microorganisms, microbial stress adaptation have been reported to influence the efficiency of PEF inhibition.
Various types of juices, milk, yogurt, and liquid egg have been tested by PEF and its benefits for their processes have been reported.
Foods are exposed to high pressure (100 to 800 MPa) during HPP for a short period, typically ranging from a few seconds to several minutes (3-5 minutes), depending on a target food temperature. Process temperature during the pressure treatment can be specified from below 0 °C to above 100 °C.


Foods are pressurized using a pressure-transmitting medium (e.g. water). In a typical HPP, the food product to be pressurized is packaged in a flexible container, such as pouches and plastic bottles, and is loaded into a high pressure chamber filled with the pressure-transmitting medium. The hydraulic fluid in the chamber is pressurized with a pump, and this pressure is transmitted through the package into the food product. The food product is uniformly treated by high pressure that is evenly distributed throughout the product. No edge or thickness effect takes place. The industrial equipments have been designed for discontinuous batch processing (capacity: from 10 to 500 L/h) of solid, viscous, and particulated foods and for semi-continuous bulk processing (capacity: from 1 to 4 ton/h) of liquid foods.
High pressure causes deprotonation of charged groups and disruption of salt bridges and hydrophoboic bonds in microbial cell membranes. These deprotonation and disruption result in conformational changes and denaturation of proteins, which causes the death of microorganisms. Generally, bacteria are more resistant to HPP than yeasts and molds.
Critical process factors include pressure, magnitude and duration of high pressure treatment, decompression time, treatment temperature, product initial temperature, product pH, product composition, type of pressurizing medium, type and number of microorganisms, type of food packaging and concurred processing aids, packaging material integrity.
HPP can be used to process both liquid and solid foods. Foods with a high acid content are particularly good candidates for HPP technology. High pressure processed products are commercially available in the United States, European, and Japanese retail markets. Examples of high-pressure processed products commercially available in the United States include fruit smoothies, fruit jellies and jams, fruit juices, tomato salsa, guacamole, cooked ready-to-eat meats, oysters, ham, chicken strips, pourable salad dressing, and rice cakes.
In aseptic packaging, food is pasteurized or sterilized while food containers are formed and/or sterilized separately and then the pasteurized/sterilized food is added to the containers in a sterile environment.
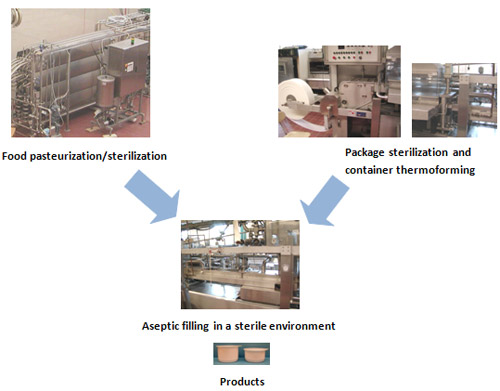
Aseptic packaging is considered most appropriate way of packaging for minimally-processed food products. Plastics and paper-laminated materials are widely used as packaging materials for aseptic food packaging. These are usually thermoformed to produce containers during the aseptic packaging process. When laminated materials are used, delamination or destructions of barrier layers (e.g. PVDC) during thermoforming must be avoided because those will negatively affect barrier and mechanical properties of thermoformed containers.


Direct surface application of antimicrobial substances onto food products by dipping, dusting, or spraying exhibits limited benefits because the active substances are neutralized on contact or diffuse rapidly from the surface into the product. Antimicrobial-incorporating films/coatings have advantages over the direct surface applications because the film can be designed to slow the antimicrobial diffusion to the surface of food. By slowing the diffusion, the substance activity at the surface of food is maintained. Thus, smaller amount of antimicrobials are needed in edible films to achieve a target shelf life as compared to dipping, dusting, or spraying agents. If an antimicrobial can be released from the film during an extended period, its activity can also be extended into the transport and storage phase of food distribution. The diffusion of the incorporated antimicrobial can be controlled by controlling film compositions.
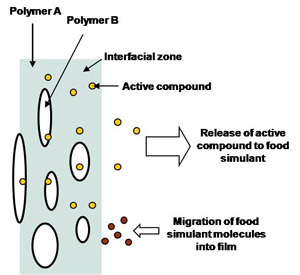
Antimicrobial films incorporating antimicrobials have advantages over those immobilizing antimicrobials at the film surface which contacts with foods. Once immobilized antimicrobials are used up, antimicrobial activities from the films are no longer expected. However, if the films incorporating antimicrobials are used, the antimicrobials at the surface of the films contacting with foods are being replenished by migration of the antimicrobials from the film matrices. Thus, minimum inhibitory concentrations (MIC) at the film surface can be maintained for an extended time with the antimicrobial films incorporating antimicrobials.
Lipid oxidation can be reduced by minimizing the O2 concentration in food. Since edible films/coatings can be excellent O2 barriers, it can reduce O2 contacting with food retarding lipid oxidation and thus reducing rancidity.
The oxidation of foods by oxygen permeated through the films/coatings can be markedly retarded by antioxidants incorporated into the films/coatings.
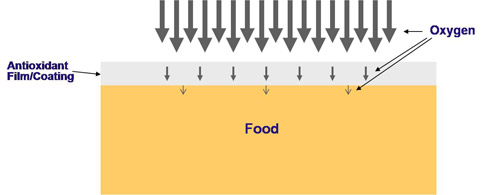
Incorporating antioxidants in edible films may have two positive effects: (1) scavenging oxygen that permeates into the film, and (2) reducing film oxygen permeability (OP).
Hazard Analysis and Critical Control Point (HACCP) is a management system used to analyze and control biological, chemical, and physical hazards that may result from harvesting, processing, manufacturing, distributing, or preparing food for consumption. The importance of HACCP has been emphasized recently due to the increase in international trade demanding worldwide of food product safety and the development of new food processing technologies.
Education and training are very important elements of the HACCP. Employees who will be responsible for the HACCP program must be trained adequately in the principles, application, and implementation of the program. Once the HACCP system is developed, commitment to its proper implementation is crucial.
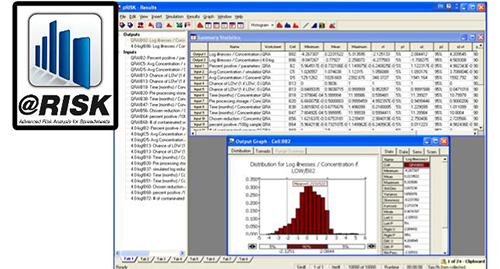
Quantitative risk assessment (QRA) is becoming a standard research tool which provides useful predictions and analyses on microbial risks and, thus, a valuable aid in implementing a HACCP system. The QRA is related to implementing HACCP by its potential involvement in identifying critical control points (CCPs), validating critical limits at a CCP, enabling rational designs of new processes and products to meet required level of safety, and evaluating processing operations for verification procedures.







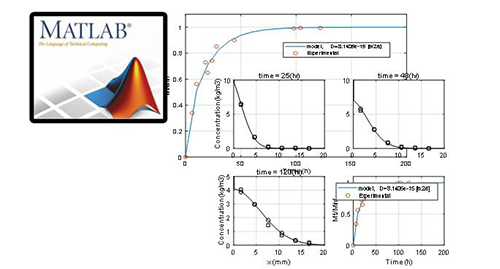
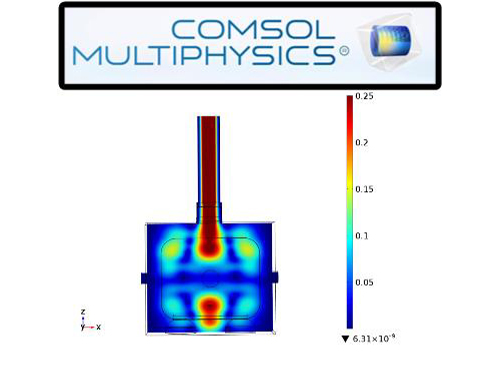
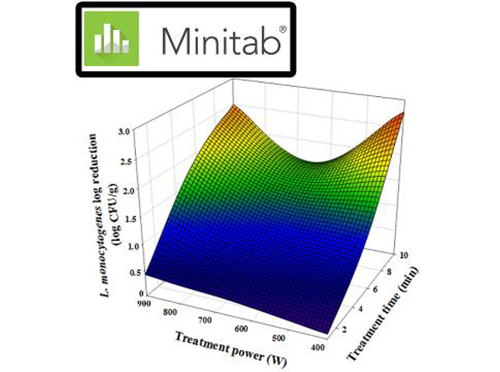
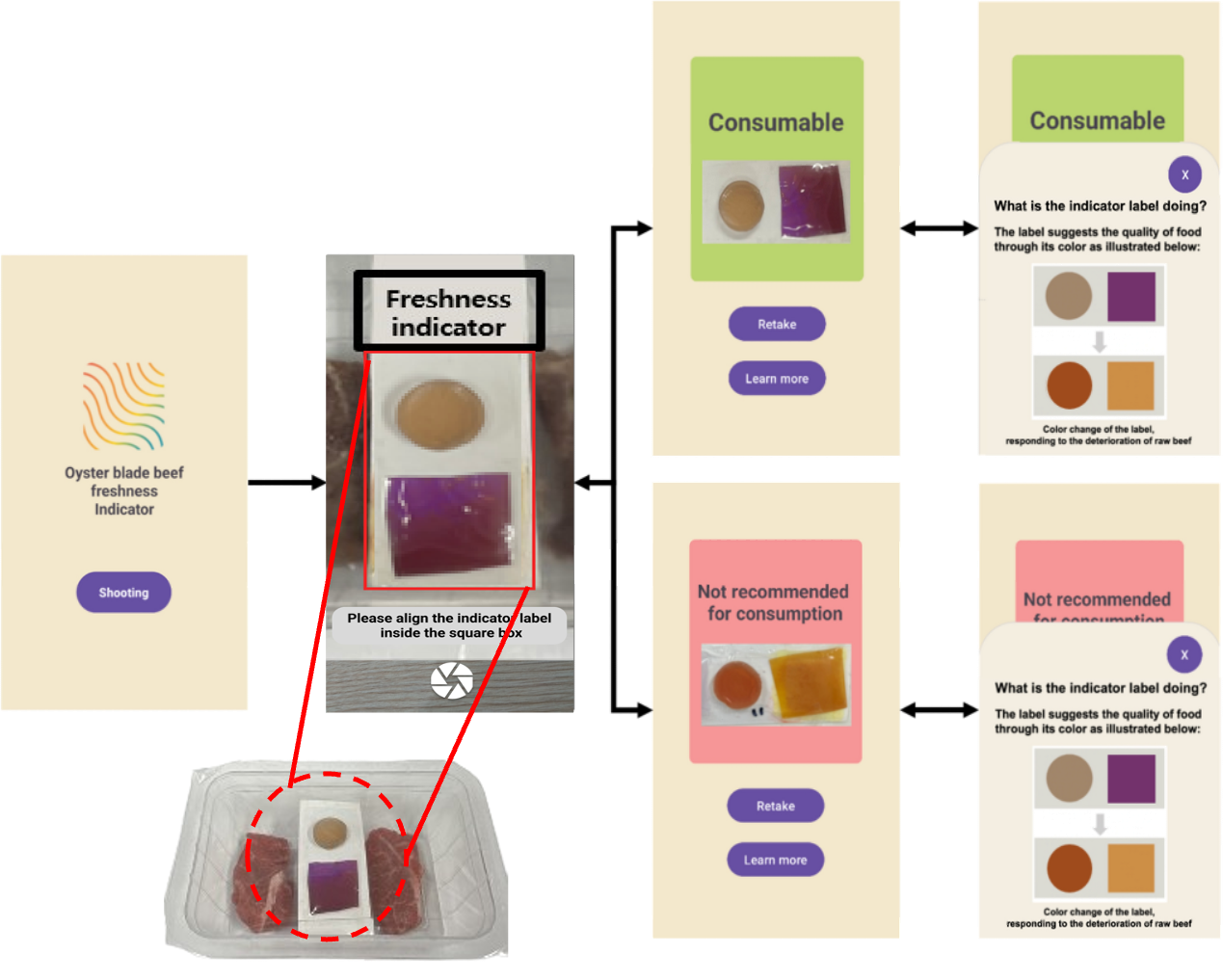
Please wait a moment.
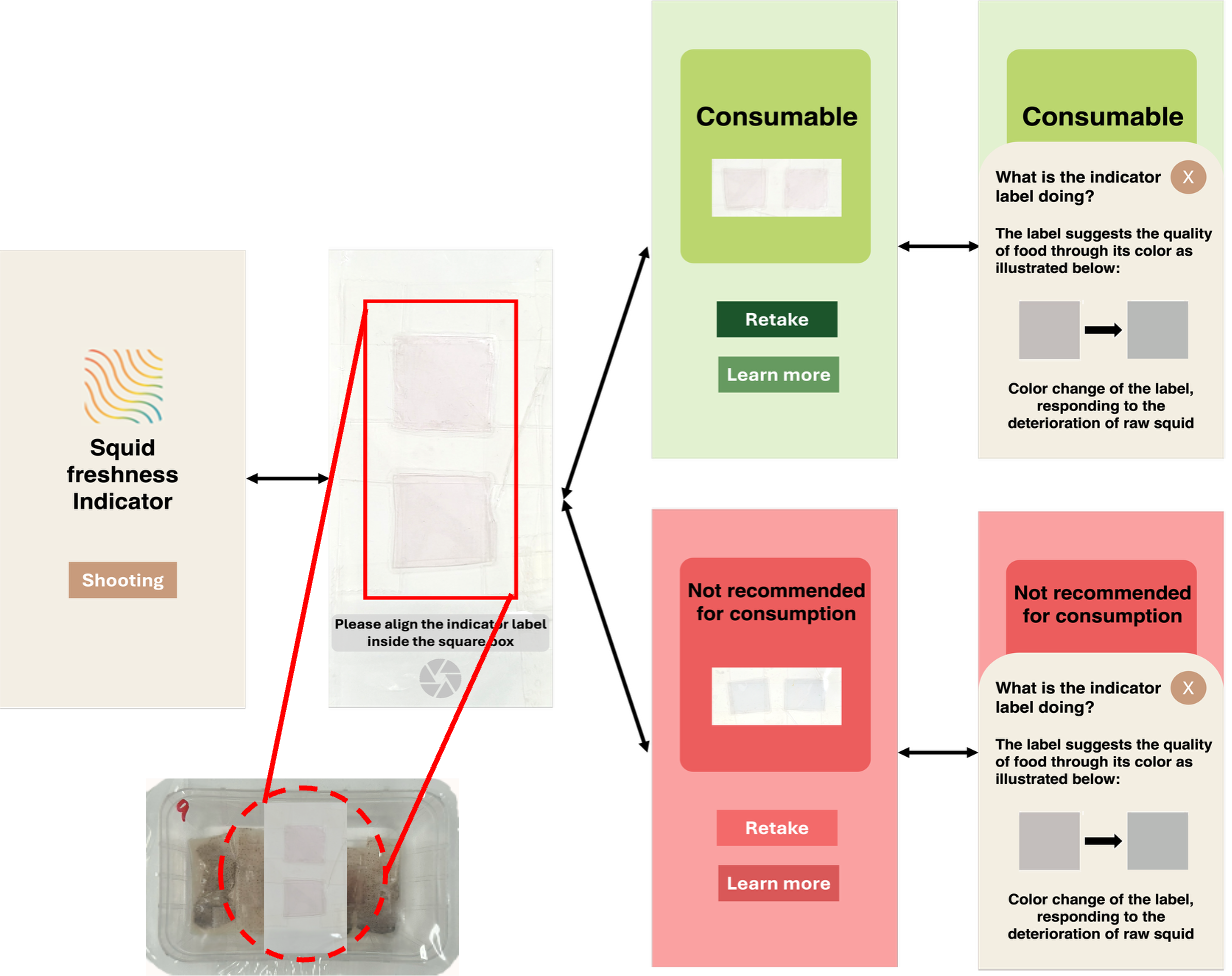
It's under preparaton.
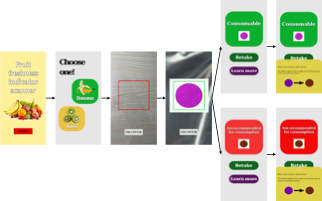
Please wait a moment.
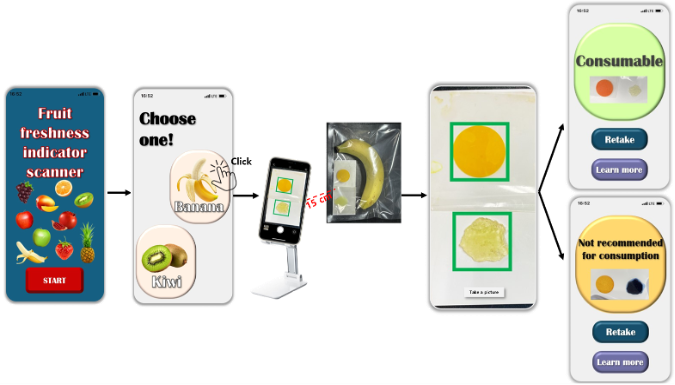
It's under preparaton.